Focus on Metastasis
Metastasis is a complex process by which tumor cells migrate from their site of origin to other organs.
For metastasis to occur, tumor cells must not only travel to new locations, but also proliferate and survive in a new environment. This challenge results in only a small percentage of circulating tumor cells forming metastases.
But when they do, this hallmark of cancer is an indicator of poor patient prognosis. And some tumor types have a higher probability of metastasis formation because they preferentially colonize specific organs for secondary tumor formation.
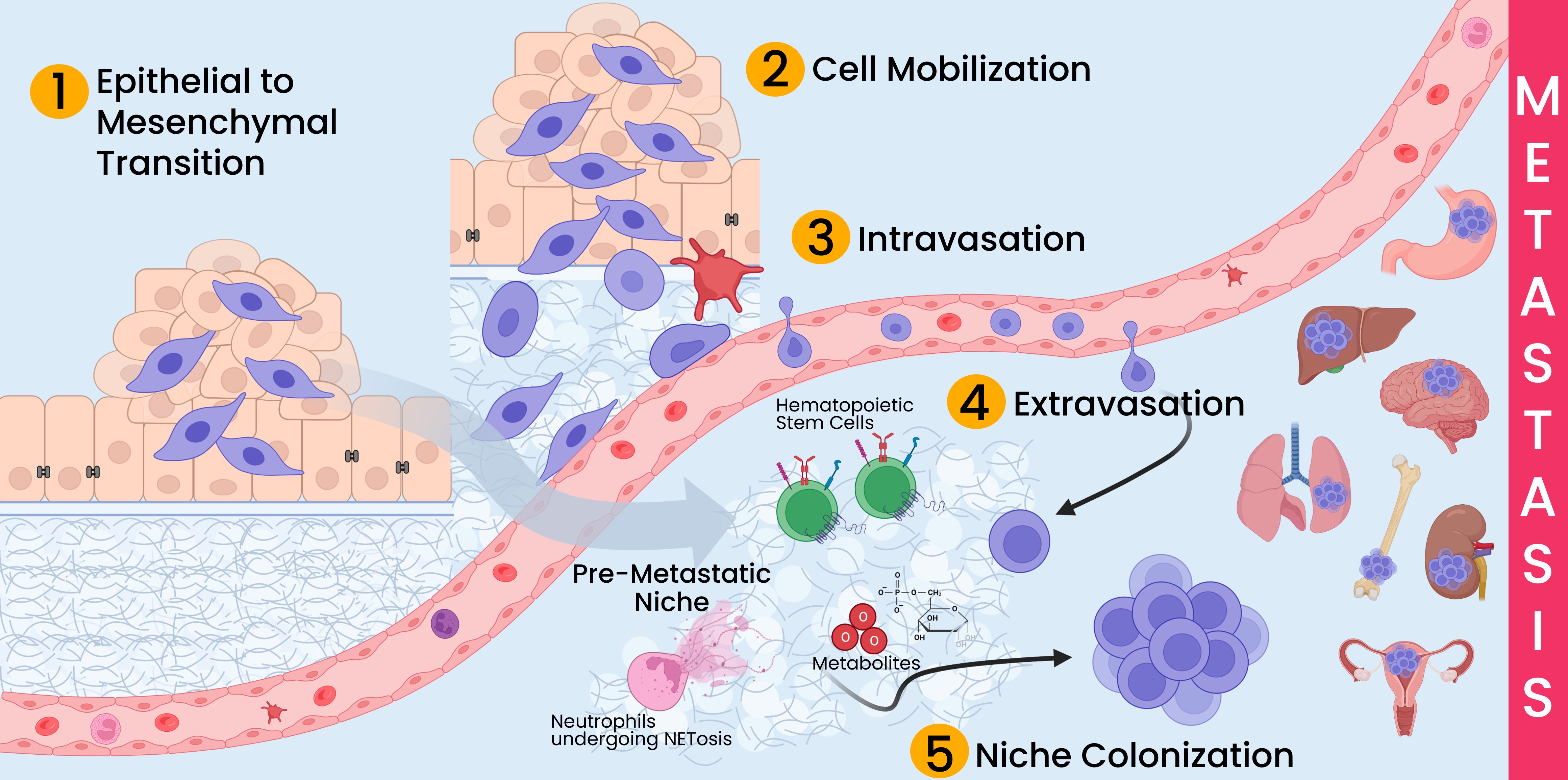
There are five basic processes that must occur to establish metastases:
- Epithelial to mesenchymal transition (EMT) describes the loss of epithelial characteristics by cancer cells and the acquisition of mesenchymal traits. These changes include loss of adhesion molecules such as E-cadherin and increase in factors contributing to cell mobility and migration like N-cadherin, vimentin, and cellular proteases.
- Cell mobilization describes the processes required for cells to escape the tumor, transverse the extracellular matrix, and locate blood vessels. The disruption of cell junctions that occurs during EMT along with additional cytoskeletal changes create cellular structures required for the movement of cells. Additionally, tumor cells can utilize pathways created by immune cells to travel the extracellular matrix and stroma to locate vasculature.
- Intravasation describes the process by which tumor cells enter blood vessels. The basement membrane surrounding endothelial cells must be at least partially degraded to allow tumor cells to squeeze between the endothelial cells and enter the lumen of the blood vessel without creating extensive damage to the vessel. Once inside the vasculature, the tumor cells can travel via active and passive mechanisms. The circulating tumor cells must survive the continual attack of the immune system and hydrostatic forces as they locate their secondary colonization site.
- Extravasation is the process tumor cells use to transit from blood vessels into the surrounding tissue. Like with intravasation, tumor cells must break through the tight junctions of the endothelium and navigate through the basement membrane and surrounding connective tissue.
- Niche colonization begins extremely early in the tumor development process, but typically is not noticeable until metastases are detected, hence the position of this step at the end of the list. The formation of premetastatic niches can happen before a primary tumor is even detectable. Primary tumor cells and cancer stem cells signal distant body sites to prime these environments for tumor growth. These pre-metastatic niches are likely established from messages sent through extracellular vesicles and other secreted cytokines and chemokines. Recent studies have found that niches where neutrophils have undergone NETosis are more likely to enable metastatic tumor growth. Eventually, these niches foster metastatic growth when primary tumor cells reach these organs.
References
- Chiang, A. C., & ¡Massagué, J. (2008). Molecular Basis of Metastasis. New England Journal of Medicine, 359(26), 2814–2823. https://doi.org/10.1056/NEJMra0805239
- Lambert, A. W., Pattabiraman, D. R., & Weinberg, R. A. (2017). Emerging Biological Principles of Metastasis. https://doi.org/10.1016/j.cell.2016.11.037
- Liu, Q., Zhang, H., Jiang, X., Qian, C., Liu, Z., & Luo, D. (2017). Factors involved in cancer metastasis: a better understanding to "seed and soil" hypothesis. Molecular Cancer, 16(1), 176. https://doi.org/10.1186/s12943-017-0742-4
- Welch, D. R., & Hurst, D. R. (2019). Defining the Hallmarks of Metastasis. Cancer Research, 79(12), 3011–3027. https://doi.org/10.1158/0008-5472.CAN-19-0458
Resources
Hypoxia Signaling: HIF1-alpha & HIF2-alpha Recombinant Rabbit Monoclonal Antibodies
The hypoxic response pathway is triggered by low levels of oxygen in the cellular environment. Hypoxia inducible transcription factor (HIF) is central to the hypoxic response. HIF exists as a heterodimeric transcription factor composed of an alpha and beta subunit. In mammals there are three HIF-alpha subunits, HIF1-alpha, HIF2-alpha, and HIF3-alpha, and one beta subunit, the aryl hydrocarbon receptor nuclear translocator (ARNT). The overexpression of HIF1-alpha and HIF2-alpha is associated with poor survival rates for various cancers. Experimental and clinical evidence strongly suggests HIF1-alpha and HIF2-alpha influence tumor development and response to treatment. Because of this, there has been major interest in developing selective HIF inhibitors; but due to the complexity of the HIF pathway, the process has been challenging. Thus, future work for therapeutic targeting of the HIFs will require a better understanding of both the HIF1-alpha and HIF2-alpha pathways.
Role of Kinesin Molecular Motor Proteins in Cancer
Kinesin superfamily proteins, or KIFs, are microtubule-dependent molecular motors whose movement is critical to a variety of cellular processes including mitosis, meiosis, and axonal transport. So far, 45 KIF genes grouped into 15 families have been identified, and at least twice as many proteins are thought to exist due to alternative splicing1. KIFs transport cargo along microtubules much like a train moves along a rail, using the energy generated from the hydrolysis of ATP to drive conformational changes that produce motility2.
Epithelial–mesenchymal Transition
Body surfaces, cavities, and the lining of hollow organs are comprised of epithelial cells. These cells are characterized by apical-basal polarity and tight cell-cell junctions, and are tightly packed together with little extracellular matrix.1 Due to these cells’ function as coverings and linings, they have one free surface not in contact with other cells, and are attached to underlying connective tissue via noncellular basement membrane.
ZEB1 interacts with ERα to drive EMT
Epithelial to mesenchymal transition (EMT) is a hallmark of cancer progression and in breast cancer not only predicts poor prognosis, but also resistance to endocrine therapy. A recent paper outlines the interactions between ZEB1 and estrogen receptor alpha and identifies an EMT hybrid population that could be targeted in novel therapeutic strategies to reverse EMT and improve patient prognosis.
USP5 drives breast cancer metastasis through HIF2α
Hypoxia is often present in solid tumors as cell growth and density increases limiting oxygen diffusion at normal levels to all the cells. While hypoxia and hypoxia inducible factors have been associated with metastasis and poor clinical prognosis, not much has been studied about the regulation of HIF2α in breast cancer. This study identifies USP5 as a novel deubiquitinase for HIF2α, increasing HIF2α stability and transcriptional activity. Together, these proteins increase cell proliferation and metastasis, and their expression positively predicts poor patient outcomes.
Sign up to receive future Focus on… features
-
Rabbit anti-p300 Antibody
Bethyl Laboratories Catalog # A300-359A A300-359A-T
 ValidatedDocuments (5) Citations ()
ValidatedDocuments (5) Citations ()Rabbit anti-p300 Antibody
Validation Performed
All Bethyl Laboratories® antibodies are validated to meet our strict performance standards.
Target: p300
Reactivity: Human
Applications:
Host: Rabbit
Clonality: Polyclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-WRN Antibody
Bethyl Laboratories Catalog # A300-238A A300-238A-T
 ValidatedDocuments (6) Citations ()
ValidatedDocuments (6) Citations ()Rabbit anti-WRN Antibody
Validation Performed
All Bethyl Laboratories® antibodies are validated to meet our strict performance standards.
Target: WRN
Reactivity: Human
Applications:
Host: Rabbit
Clonality: Polyclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-4EBP1 Antibody
Bethyl Laboratories Catalog # A300-501A A300-501A-T
 ValidatedDocuments (4) Citations ()
ValidatedDocuments (4) Citations ()Rabbit anti-4EBP1 Antibody
Validation Performed
All Bethyl Laboratories® antibodies are validated to meet our strict performance standards.
Target: 4EBP1
Reactivity: Human
Applications:
Host: Rabbit
Clonality: Polyclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-Septin 9 Antibody
Bethyl Laboratories Catalog # A302-353A A302-353A-T
 ValidatedDocuments (4) Citations ()
ValidatedDocuments (4) Citations ()Rabbit anti-Septin 9 Antibody
Validation Performed
All Bethyl Laboratories® antibodies are validated to meet our strict performance standards.
Target: Septin 9
Reactivity: Human
Applications:
Host: Rabbit
Clonality: Polyclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Mouse anti-MOV10 Monoclonal Antibody [15C1B8]
Bethyl Laboratories Catalog # A500-009A-T A500-009A A500-009ACF
 ValidatedDocuments (6) Citations ()
ValidatedDocuments (6) Citations ()Mouse anti-MOV10 Monoclonal Antibody [15C1B8]
Validation Performed
All Bethyl Laboratories® antibodies are validated to meet our strict performance standards.
Target: MOV10
Reactivity: Human, Mouse
Applications:
Host: Mouse
Clonality: Monoclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
Bulk Request